Study Guide For Medication Treatment Schizophrenia Spectrum And Other Psychosis Disorders Nurs 6630

Walden University Study Guide For Medication Treatment Schizophrenia Spectrum And Other Psychosis Disorders Nurs 6630-Step-By-Step Guide
This guide will demonstrate how to complete the Walden University Study Guide For Medication Treatment Schizophrenia Spectrum And Other Psychosis Disorders Nurs 6630 assignment based on general principles of academic writing. Here, we will show you the A, B, Cs of completing an academic paper, irrespective of the instructions. After guiding you through what to do, the guide will leave one or two sample essays at the end to highlight the various sections discussed below.
How to Research and Prepare for Study Guide For Medication Treatment Schizophrenia Spectrum And Other Psychosis Disorders Nurs 6630
Whether one passes or fails an academic assignment such as the Walden University Study Guide For Medication Treatment Schizophrenia Spectrum And Other Psychosis Disorders Nurs 6630 depends on the preparation done beforehand. The first thing to do once you receive an assignment is to quickly skim through the requirements. Once that is done, start going through the instructions one by one to clearly understand what the instructor wants. The most important thing here is to understand the required format—whether it is APA, MLA, Chicago, etc.
After understanding the requirements of the paper, the next phase is to gather relevant materials. The first place to start the research process is the weekly resources. Go through the resources provided in the instructions to determine which ones fit the assignment. After reviewing the provided resources, use the university library to search for additional resources. After gathering sufficient and necessary resources, you are now ready to start drafting your paper.
How to Write the Introduction for Study Guide For Medication Treatment Schizophrenia Spectrum And Other Psychosis Disorders Nurs 6630
The introduction for the Walden University Study Guide For Medication Treatment Schizophrenia Spectrum And Other Psychosis Disorders Nurs 6630 is where you tell the instructor what your paper will encompass. In three to four statements, highlight the important points that will form the basis of your paper. Here, you can include statistics to show the importance of the topic you will be discussing. At the end of the introduction, write a clear purpose statement outlining what exactly will be contained in the paper. This statement will start with “The purpose of this paper…” and then proceed to outline the various sections of the instructions.

Struggling to Meet Your Deadline?
Get your assignment on Study Guide For Medication Treatment Schizophrenia Spectrum And Other Psychosis Disorders Nurs 6630 done on time by medical experts. Don’t wait – ORDER NOW!
How to Write the Body for Study Guide For Medication Treatment Schizophrenia Spectrum And Other Psychosis Disorders Nurs 6630
After the introduction, move into the main part of the Study Guide For Medication Treatment Schizophrenia Spectrum And Other Psychosis Disorders Nurs 6630 assignment, which is the body. Given that the paper you will be writing is not experimental, the way you organize the headings and subheadings of your paper is critically important. In some cases, you might have to use more subheadings to properly organize the assignment. The organization will depend on the rubric provided. Carefully examine the rubric, as it will contain all the detailed requirements of the assignment. Sometimes, the rubric will have information that the normal instructions lack.
Another important factor to consider at this point is how to do citations. In-text citations are fundamental as they support the arguments and points you make in the paper. At this point, the resources gathered at the beginning will come in handy. Integrating the ideas of the authors with your own will ensure that you produce a comprehensive paper. Also, follow the given citation format. In most cases, APA 7 is the preferred format for nursing assignments.
How to Write the Conclusion for Study Guide For Medication Treatment Schizophrenia Spectrum And Other Psychosis Disorders Nurs 6630
After completing the main sections, write the conclusion of your paper. The conclusion is a summary of the main points you made in your paper. However, you need to rewrite the points and not simply copy and paste them. By restating the points from each subheading, you will provide a nuanced overview of the assignment to the reader.
How to Format the References List for Study Guide For Medication Treatment Schizophrenia Spectrum And Other Psychosis Disorders Nurs 6630
The very last part of your paper involves listing the sources used in your paper. These sources should be listed in alphabetical order and double-spaced. Additionally, use a hanging indent for each source that appears in this list. Lastly, only the sources cited within the body of the paper should appear here.
Stuck? Let Us Help You
Completing assignments can sometimes be overwhelming, especially with the multitude of academic and personal responsibilities you may have. If you find yourself stuck or unsure at any point in the process, don’t hesitate to reach out for professional assistance. Our assignment writing services are designed to help you achieve your academic goals with ease.
Our team of experienced writers is well-versed in academic writing and familiar with the specific requirements of the Study Guide For Medication Treatment Schizophrenia Spectrum And Other Psychosis Disorders Nurs 6630 assignment. We can provide you with personalized support, ensuring your assignment is well-researched, properly formatted, and thoroughly edited. Get a feel of the quality we guarantee – ORDER NOW.
A Sample Answer For the Assignment: Study Guide For Medication Treatment Schizophrenia Spectrum And Other Psychosis Disorders Nurs 6630
Mental illness is known to have various negative impacts on patients’ lives. Among them are schizophrenia spectrum and other psychosis disorders (Jester et al.,2023). These illnesses usually result in undesirable symptoms like delusions, hallucinations, disorganized thinking or speech, and disorganized or abnormal motor behaviors. As such, the PMHNPs should possess sufficient knowledge concerning these conditions and their symptoms for accurate diagnosis, treatment, and management. Study guides can play a critical role in enhancing a person’s ability to learn about these conditions and their management using antipsychotic agents. Therefore, the purpose of this assignment is to create a study guide that presents the medication treatment for schizophrenia spectrum and other psychosis disorders.
Drug Description
The chosen medication for this study guide is Quetiapine. This mediation has widely been used in treating schizophrenia (de Miranda et al.,2020). The brand name is Seroquel. The FDA approved this medication to be used in treating schizophrenia, major depressive disorder, and bipolar disorder.
Non-FDA uses
Apart from the FDA indications, Seroquel has also been used for non-FDA-approved functions. The non-FDA uses include the following.
- Treatment of insomnia where low doses are usually given
- Treatment of alcohol dependence as heavy drinkers has been observed to reduce their drinking tendencies when using the medication (de Miranda et al.,2020)
- It is also used in the treatment of general anxiety disorder, where lower doses are used.
Drug classification
Seroquel is an antipsychotic under second-generation antipsychotics.
| Mechanism of action | Pharmacokinetics | Pharmacodynamics |
| It is known to act on the dopaminergic D1 and D2 receptors. It is also an antagonist for serotonin receptors and D2 receptors. | Absorption: An immediate and extended release of 1.5 hours and 6 hours, respectively (Peak plasma time) | The medication blocks the dopamine receptors to help lower incidences of delusions and hallucinations caused by schizophrenia. |
| Acts on dopaminergic D1 and D2 receptors. | Metabolism: The medication metabolism takes place in the liver by CYP3A4 (Badhan & Macfarlane, 2020) Elimination: 20% of elimination is through feces, while 73% is through excretion. |
Recommended dosing:
- 150-750 mg/day (Immediate release); 400-800 mg/day (extended-release).
- Children below twelve years: No safety has been established.
- Children above twelve years: 400-800 mg/day
- Not recommended for breastfeeding and pregnant women
- Geriatrics: 50-200 mg/day (Immediate); 50 mg/day (Extended) (de Miranda et al.,2020)
Route of Administration: The medication is taken through the mouth
Dosing alterations consideration: individuals susceptible to hypotensive reactions and the elderly.
Half-life: This is the time which is taken by a medication’s concentration to reduced by half of the original concentration in the body. It is important to understand the half-life of medication since it dictates the steady-state concentration and excretion. The half-life for Seroquel is six hours for immediate release and seven hours for extended-release (de Miranda et al.,2020).
Side Effects and Possible Adverse Reactions
The medication is connected to various side effects and possible adverse reactions, which might be a concern to patients. It is important that a patient is educated regarding the same. They are illustrated below.
Contraindications For Use Including Significant Drug-To-Drug Interactions
The medication is contraindicated for patients with hypersensitivity. The medication has been shown to pause and increase mortality risk in elderly individuals displaying dementia-connected psychosis. It may interact with medication that, leads to prolonged QT intervals. The medication specifically interacts with Leuprolide, Lefamulin, Goserelin, and Amisupride (Oruch et al.,2020).
Overdose Considerations
- The medication can lead to death in cases of overdose. Indeed, toxicity may happen with levels higher than 1500 ng/mL.
- Supportive care has widely been used in treating Seroquel overdose.
- Measures for acute toxicity include ventilation, sufficient oxygenation, and maintaining the airway (Oruch et al.,2020).
- Gastric lavage and administration of activated charcoal with a laxative can also help in stopping the absorption of the drug if administered.
Diagnostics and labs monitoring
Comorbidities considerations
- Practitioners should monitor the blood glucose levels of patients with diabetes mellitus to limit the chances of hyperosmolar coma (Osborne et al.,2020).
- Practitioners should be careful with individuals with hypomagnesemia, cardiac arrhythmia, and hypokalemia and should get a metabolic panel before they can start administering the medication.
Legal and ethical considerations
- Health practitioners have to uphold the patient’s health information confidentiality as a way of preventing legal issues (Limandri, 2019)
- It is also important to uphold beneficence by ensuring that administered medication leads to optimum benefits when treating the approved indications for the medication.
- The practitioners should also consider the side effects of the medication and ensure that the expected benefits outweigh the potential risks hence upholding nonmaleficence.
- Consent is also required. Therefore, the health practitioners should obtain consent from the patients and ensure that the patient is well informed regarding the potential side effects and adverse effects of Seroquel.
- Pertinent Patient Education Considerations
It is important to explore pertinent patient education considerations when administering Seroquel. As such, the practitioner has to educate the patient regarding various aspects, such as side effects, potential benefits, and indications. The patients should be educated on the need to adhere to the medication regimen and ensure that they see they visit the facility in case of any adverse effects or reactions (de Miranda et al.,2020).
. In addition, the patient also needs to be informed of the instances when the medication can be discontinued.
Conclusion
This study guide has explored the use of Seroquel, which is an FDA-approved medication for treating schizophrenia, major depressive disorder, and bipolar disorder. The medication also has various non-FDA-approved uses, such as the treatment of insomnia and generalized anxiety disorder. The guideline has also explored various aspects such as indications for use, contraindication, potential side effects and adverse reactions, diagnostics and lab monitoring, and various ethical principles to consider.
References
Badhan, R. K., & Macfarlane, H. (2020). Quetiapine dose optimisation during gestation: A pharmacokinetic modelling study. Journal of Pharmacy and Pharmacology, 72(5), 670–681. https://doi.org/10.1111/jphp.13236
de Miranda, A. S., Ferreira, R. N., Teixeira, A. L., & de Miranda, A. S. (2020). Mood Stabilizers: Quetiapine. NeuroPsychopharmacotherapy, 1-23.
Jester, D. J., Thomas, M. L., Sturm, E. T., Harvey, P. D., Keshavan, M., Davis, B. J., … & Jeste, D. V. (2023). Review of major social determinants of health in schizophrenia-spectrum psychotic disorders: I. Clinical outcomes. Schizophrenia Bulletin, 49(4), 837–850. https://doi.org/10.1093/schbul/sbad023
Limandri, B. J. (2019). Ethical Reasoning in Prescribing and Monitoring Psychotropic Medications. Journal of Psychosocial Nursing and Mental Health Services, 57(1), 7-10. https://doi.org/10.3928/02793695-20181212-03
Oruch, R., Pryme, I., Fasmer, O., & Lund, A. (2020). Quetiapine: An objective evaluation of pharmacology, clinical uses and intoxication. EC Pharmacol Toxicol, 8, 1-26. https://elibrary.ru/item.asp?id=42474663
Osborne, V., Davies, M., Evans, A., & Shakir, S. (2020). Observational assessment of safety in Seroquel (OASIS): a specialist cohort event monitoring (SCEM) study in England. Therapeutic advances in psychopharmacology, 10, 2045125320954616. https://doi.org/10.1177/2045125320954616
Description Of the Psychopharmacological Medication Agent
Cariprazine is a psychopharmacological drug sold under the brand name Vraylar. It is an adult atypical antipsychotic drug of the second generation prescribed to treat schizophrenia and bipolar disorder. Cariprazine acts by altering dopamine and serotonin quantities in the brain, which alleviates symptoms including mania, hallucinations, and disordered thinking. The drug is offered as tablets and is typically ingested once daily.
Cariprazine has been given FDA approval for the management of schizophrenia and adult-onset bipolar disorder. Cariprazine is recommended for schizophrenia as a monotherapy or as an additional medication to lessen relapse frequency (Pinto et al., 2019). In individuals with bipolar I disorder who have responded to initial therapy, cariprazine is recommended for the acute management of manic or mixed episodes and for the maintenance of a calm mood.
Research for Non-FDA Uses
One area of research interest has been the use of cariprazine for the treatment of major depressive disorder (MDD). Several clinical trials have been conducted to evaluate the efficacy and safety of cariprazine as an adjunctive treatment for MDD, with some studies showing promising results. A study found that cariprazine as an adjunctive treatment to an antidepressant was effective in reducing depressive symptoms in patients with MDD who had not responded to prior antidepressant treatment (Pinto et al., 2019).
Another potential non-FDA use of cariprazine is for the treatment of substance use disorders. Preclinical studies have suggested that cariprazine may be effective in reducing drug-seeking behavior and reinstatement of drug use in animal models of addiction. Additionally, a pilot study published in the Journal of Dual Diagnosis in 2018 found that cariprazine may be useful for the treatment of cocaine use disorder in humans.
Drug Classification
Cariprazine is classified as a second-generation atypical antipsychotic medication. This class of drugs is used to treat various psychiatric disorders, including schizophrenia and bipolar disorder, by modulating the levels of dopamine and other neurotransmitters in the brain (Pinto et al., 2019). Second-generation atypical antipsychotics are considered to be a newer class of antipsychotic drugs compared to first-generation antipsychotics, and they have been developed to offer better efficacy and fewer side effects.
Cariprazine is structurally similar to aripiprazole, another atypical antipsychotic medication. Both medications are partial agonists at the dopamine D2 receptor and the serotonin 5-HT1A receptor, which means that they can stimulate or inhibit these receptors depending on the level of activity of the neurotransmitter. This unique pharmacological profile is thought to contribute to the efficacy and tolerability of cariprazine in the treatment of psychiatric disorders.
Mechanism of Action
Cariprazine’s mechanism of action is complex and involves modulation of multiple neurotransmitter systems in the brain, including dopamine and serotonin. Specifically, cariprazine acts as a partial agonist at dopamine D2 and D3 receptors and a partial agonist at serotonin 5-HT1A receptors, while also acting as an antagonist at serotonin 5-HT2A receptors (Laszlovszky et al., 2021).

The partial agonist activity at dopamine D2 and D3 receptors allows cariprazine to modulate the dopaminergic pathways in the brain, which are known to be involved in the pathophysiology of schizophrenia and bipolar disorder. By acting as a partial agonist, cariprazine can both stimulate and inhibit these receptors, depending on the level of dopamine activity in the brain. This unique pharmacological profile is thought to contribute to the medication’s ability to improve symptoms such as delusions, hallucinations, and disorganized thinking.
In addition to its effects on dopamine, cariprazine also has partial agonist activity at serotonin 5-HT1A receptors, which are involved in the regulation of mood, anxiety, and cognition. By modulating the activity of these receptors, cariprazine may help to improve symptoms of depression and anxiety that are commonly associated with schizophrenia and bipolar disorder.
Pharmacokinetics and Pharmacodynamics
Cariprazine is an orally administered medication that is rapidly absorbed by the body, with peak plasma concentrations occurring within 3-4 hours of ingestion. The bioavailability of cariprazine is estimated to be approximately 52%, which means that about half of the medication reaches systemic circulation following oral administration (Laszlovszky et al., 2021).
Once absorbed, cariprazine is extensively metabolized in the liver by enzymes such as cytochrome P450 3A4 (CYP3A4) and cytochrome P450 2D6 (CYP2D6). The metabolites of cariprazine are primarily eliminated through urine and feces, with approximately 26% of the dose being eliminated in urine and 51% in feces.
Cariprazine acts as an antagonist at serotonin 5-HT2A receptors, which are involved in the regulation of sensory perception, cognition, and mood. By blocking the activity of these receptors, cariprazine may help to reduce the risk of side effects such as hallucinations and agitation that are associated with other antipsychotic medications.
Appropriate Dosing, Administration Route, and any Considerations for Dosing Alterations
The recommended starting dose for cariprazine in the treatment of schizophrenia is 1.5 mg/day, taken orally with or without food. The dose may be increased gradually over several days to a target dose of 4.5 mg/day, based on the patient’s response and tolerability. The maximum recommended dose is 6 mg/day.
For the treatment of bipolar disorder, the recommended starting dose is 1.5 mg/day, taken orally with or without food (Fagiolini et al., 2020). The dose may be increased gradually over several days to a target dose of 3 mg/day, based on the patient’s response and tolerability. The maximum recommended dose is 6 mg/day.
Cariprazine is available in tablet form and should be taken orally once daily, before or after meals. The tablets should be swallowed whole and should not be crushed or chewed (Fagiolini et al., 2020). Cariprazine may interact with other medications that affect the metabolism of the medication, such as CYP3A4 and CYP2D6 inhibitors or inducers. Patients taking these medications may require a dose adjustment of cariprazine to ensure optimal efficacy and tolerability.
Considerations Of Use and Dosing in Specific Specialty Populations
Children and adolescents
Not approved for use in children and adolescents under the age of 18 years old.
Elderly
Elderly patients may be more sensitive to the effects of cariprazine due to changes in metabolism and clearance of the medication (Fagiolini et al., 2020). Therefore, the dose may need to be adjusted in elderly patients to avoid adverse effects such as sedation, orthostatic hypotension, or extrapyramidal symptoms.
Pregnancy and breastfeeding
Cariprazine should only be used during pregnancy or breastfeeding if the potential benefits to the mother outweigh the potential risks to the fetus or infant.
Suicidal behaviors
May increase the risk of suicidal thoughts or behaviors, especially in children and young adults. Patients should be closely monitored for signs of suicidal ideation, especially during the first few months of treatment or after a change in dose.
Half-life
Half-life is the amount of time it takes for half of the initial dose of a drug to be eliminated from the body. The half-life of a medication is important because it determines the frequency and timing of dosing and can affect the drug’s efficacy and potential for adverse effects (Fagiolini et al., 2020). The half-life of cariprazine is approximately 2-4 days, meaning it takes 2-4 days for half of the initial dose of cariprazine to be eliminated from the body.
This relatively long half-life suggests that cariprazine may be suitable for once-daily dosing, but it may take longer to reach a steady state and may require a longer time to clear from the body if dosing is discontinued. The half-life of cariprazine should be taken into account when determining appropriate dosing regimens and monitoring for potential adverse effects.
Side effects
Common side effects of cariprazine include:
- Restlessness
- Extrapyramidal symptoms (such as tremors or muscle stiffness)
- Sedation
- Nausea
- Vomiting
- Constipation
- Headache
- Weight gain
Contraindications for use including significant drug to drug interactions
Contraindications for the use of cariprazine include:
- Hypersensitivity to cariprazine or any component of the formulation
- Unstable heart disease
- QTc prolongation
- Severe hepatic impairment
- Severe renal impairment
- Known history of prolonged QT syndrome
Significant drug-to-drug interactions include:
- Strong CYP3A4 inhibitors can increase the concentration of cariprazine in the body, potentially leading to increased side effects (Edinoff et al., 2020).
- Strong CYP3A4 inducers (such as rifampin and phenytoin) can decrease the concentration of cariprazine in the body, potentially reducing its efficacy.
Overdose Considerations
An overdose of cariprazine can be dangerous and potentially life-threatening. Symptoms of an overdose may include severe sedation, seizures, confusion, cardiac arrhythmias, and respiratory depression (Edinoff et al., 2020). In the event of an overdose, immediate medical attention should be sought.
Diagnostics and labs monitoring
- Complete blood count
- Liver function tests
- Electrolyte levels
Comorbidities Considerations
Cardiovascular disease: Cariprazine can cause changes in blood pressure and heart rate, so caution is advised in patients with cardiovascular disease. Blood pressure and heart rate should be monitored regularly during treatment.
Diabetes: Cariprazine can cause changes in blood glucose levels, so caution is advised in patients with diabetes. Blood glucose levels should be monitored regularly during treatment.
Seizure disorder: Cariprazine can lower the seizure threshold, so caution is advised in patients with a history of seizures or epilepsy (Edinoff et al., 2020).
Renal or hepatic impairment: Cariprazine is metabolized in the liver and excreted in the kidneys, so caution is advised in patients with renal or hepatic impairment. Dose adjustments may be necessary.
Substance use disorder: Cariprazine may have potential for abuse or dependence, so caution is advised in patients with a history of substance use disorder.
Pregnancy or breastfeeding: The safety of cariprazine during pregnancy and breastfeeding is not well-established, and the potential risks and benefits should be carefully considered before prescribing to women who are pregnant or breastfeeding.
Legal and Ethical Considerations
One of the most critical legal considerations is obtaining informed consent from patients. Healthcare providers must explain the potential benefits and risks of cariprazine, as well as any alternative treatments that may be available. Informed consent is critical to ensuring that patients understand the implications of taking medication, and that they are fully informed about their treatment options. Another legal consideration is the off-label use of cariprazine.
While cariprazine is approved by the FDA for the treatment of schizophrenia and bipolar disorder, healthcare providers may also prescribe it for off-label uses (Rancans et al., 2021). Off-label use is legal, but it must be based on clinical judgment and the best available evidence. Providers should also be aware of any potential legal risks associated with off-label use.
Prescribing practices are also essential legal and ethical considerations. Healthcare providers must prescribe cariprazine at the appropriate dosage and monitor patients for side effects or adverse reactions. Providers should also follow established medical guidelines and best practices when prescribing cariprazine or any other medication.
Healthcare providers must protect patient privacy by following all relevant privacy laws and regulations, such as HIPAA. This includes keeping patient medical records confidential and only sharing information with other healthcare providers on a need-to-know basis. Finally, healthcare providers may be held liable for any harm that their patients experience as a result of medication errors or other negligent actions.
Pertinent Patient Education Considerations
Dosing and administration
Patients should be educated on how to take cariprazine, including the appropriate dosage and administration route. Patients should also be advised to take the medication as directed by their healthcare provider and not to adjust their dose or stop taking the medication without consulting their provider.
Side effects and adverse reactions
Patients should be informed about the potential side effects and adverse reactions of cariprazine. They should be advised to contact their healthcare provider immediately if they experience any side effects, such as dizziness, nausea, or constipation.
Drug interactions
Patients should be informed of potential drug interactions with cariprazine, including over-the-counter medications, herbal supplements, and other prescription drugs. Patients should be advised to consult their healthcare provider before taking any new medications (Rancans et al., 2021).
Importance of compliance
Patients should be educated on the importance of compliance with their cariprazine treatment regimen. Skipping doses or discontinuing treatment without consulting their healthcare provider can lead to a worsening of their condition.
Pregnancy and breastfeeding
Patients should be informed about the potential risks of taking cariprazine during pregnancy or while breastfeeding. Patients who are pregnant or breastfeeding should consult their healthcare provider before taking cariprazine.
Suicide risk
Patients should be advised of the potential risk of suicide associated with cariprazine and other medications used to treat mental illness. Patients should be advised to contact their healthcare provider immediately if they experience any suicidal thoughts or behaviors.
References
Correll, C. U., & Schooler, N. R. (2020). Negative symptoms in schizophrenia: a review and clinical guide for recognition, assessment, and treatment. Neuropsychiatric Disease and Treatment, 519-534. Doi: 10.2147/NDT.S225643
Edinoff, A., Ruoff, M. T., Ghaffar, Y. T., Rezayev, A., Jani, D., Kaye, A. M., … & Urits, I. (2020). Cariprazine to treat schizophrenia and bipolar disorder in adults. Psychopharmacology Bulletin, 50(4), 83. https://doi.org/10.1211/pj.2015.20069435
Fagiolini, A., Alcalá, J. Á., Aubel, T., Bienkiewicz, W., Bogren, M. M., Gago, J., Cerveri, G., Colla, M., Sanchez, F. C., Cuomo, A., Helge, F., Iacoponi, E., Karlsson, P., Peddu, P., Pettorruso, M., Pereira, H. J., Schölin, J. S., & Vernaleken, I. B. (2020). Treating schizophrenia with cariprazine: From clinical research to clinical practice. Real world experiences and recommendations from an international panel. Annals of General Psychiatry, 19(1). https://doi.org/10.1186/s12991-020-00305-3
Laszlovszky, I., Barabássy, Á., & Németh, G. (2021). Cariprazine, a broad-spectrum antipsychotic for the treatment of schizophrenia: Pharmacology, efficacy, and safety. Advances in Therapy, 38(7), 3652-3673. https://doi.org/10.1007/s12325-021-01797-5
Pinto, J. V., Saraf, G., Vigo, D., Keramatian, K., Chakrabarty, T., & Yatham, L. N. (2019). Cariprazine in the treatment of bipolar disorder: A systematic review and meta‐analysis. Bipolar Disorders, 22(4), 360-371. https://doi.org/10.1111/bdi.12850
Rancans, E., Dombi, Z. B., Mátrai, P., Barabássy, Á., Sebe, B., Skrivele, I., & Németh, G. (2021). The effectiveness and safety of cariprazine in schizophrenia patients with negative symptoms and insufficient effectiveness of previous antipsychotic therapy: An observational study. International Clinical Psychopharmacology, 36(3), 154-161. https://doi.org/10.1097/yic.0000000000000351
Antipsychotic agents, also called neuroleptic or major tranquilizers, are used primarily to treat schizophrenia. Schizophrenia is characterized primarily by a clear sensory but marked thinking disturbance. Second-generation/ Atypical antipsychotics are widely used due to their broad spectrum of receptor activity since they affect Serotonin, dopamine, and GABA neurotransmitters (de Miranda et al., 2020). Besides, they are better at alleviating negative symptoms and cognitive dysfunction than typical antipsychotics. The purpose of this assignment is to develop a study guide for an antipsychotic agent.
Drug Description
Quetiapine, whose brand name goes by Seroquel, is used in treating schizophrenia. It is FDA-approved for treating schizophrenia, Bipolar disorder, and major depressive disorder (MDD) as an adjunctive treatment (de Miranda et al., 2020).
Click here to ORDER an A++ paper from our Verified MASTERS and DOCTORATE WRITERS: STUDY GUIDE FOR MEDICATION TREATMENT SCHIZOPHRENIA SPECTRUM AND OTHER PSYCHOSIS DISORDERS NURS 6630
Non-FDA uses
The non-FDA uses of Seroquel include the treatment of generalized anxiety disorder (GAD), Alcohol Dependence, and Insomnia.
- According to Ansara (2020), Seroquel exhibits efficacy in managing treatment-resistant-GAD as an adjunctive agent. In this case, smaller doses than those prescribed for schizophrenia and bipolar disorder are usually needed for symptom improvement.
- Seroquel has been found to reduce alcohol consumption in heavy drinkers and has the potential for treatment for alcohol dependence, particularly among heavy drinkers (Vatsalya et al., 2020).
- Low doses of quetiapine are usually prescribed for insomnia, although this is a non-FDA use due to potential adverse effects like weight gain and akathisia (Boafo et al., 2020).
Drug classification
Seroquel is an antipsychotic under second-generation antipsychotics.
| MOA | Pharmacokinetics | Pharmacodynamics |
| An antagonist for D2 receptors and serotonin receptors. | Absorption: Bioavailability: 100% Peak plasma time: Immediate release-1.5 hr; extended release-6 hrs | Reduces the hallucinations and delusions associated with schizophrenia by blocking dopamine receptors in the mesolimbic system of the brain. |
| Acts on dopaminergic D1 and D2 receptors. | Metabolism: Metabolized in the liver by CYP3A4 | |
| Elimination: Excretion: Urine (73%), feces (20%). |
Appropriate dosing: 150-750 mg/day (Immediate release); 400-800 mg/day (extended release).
Children <12 years: safety not established.
Children >12 years: Dose range 400-800 mg/day (de Miranda et al., 2020).
Geriatrics: 50-200 mg/day (Immediate); 50 mg/day (Extended)
Pregnant and breastfeeding women: Not recommended.
Route of Administration: Orally.
Considerations for dosing alterations: Elderly and patients predisposed to hypotensive reactions.
Half-life: The time it takes for the concentration of a drug to decrease to half of its initial dose in the body.
- Understanding half-life is important because it determines a drug’s excretion rates and steady-state concentrations. After one half-life has passed, half of the starting drug amount is eliminated from the body (Smith et al., 2018).
- Seroquel has a half-life of 6 hours for immediate release formulation and 7 hours for extended-release formulation.
Side effects/adverse reaction potentials
Seroquel is associated with various adverse effects, including somnolence fatigue, dry mouth, constipation, increased appetite, weight gain, orthostatic hypertension, and dizziness (de Miranda et al., 2020). Neuroleptic malignant syndrome is a possible adverse effect due to the drug’s D2 receptor blockage.
Contraindications for use including significant drug-to-drug interactions
- Currently, no identified FDA contraindications of quetiapine.
- It is contraindicated in patients with documented hypersensitivity.
- However, quetiapine is associated with an increased risk of death in elderly patients with dementia-related psychosis (Osborne et al., 2020).
- Precaution is needed with drugs that prolong QT intervals and patients with prolonged QT intervals.
Contraindications due to drug-to-drug interactions
- Amisulpride
- Goserelin
- Lefamulin
- Leuprolide
Overdose Considerations
Seroquel can be life-threatening if taken in an overdose. Toxicity occurs with levels > 1500 ng/mL.
Supportive care is the mainstay of treatment in an overdose.
Measures for acute toxicity include: Maintaining the airway; Ensuring adequate oxygenation; Ventilation (Osborne et al., 2020).
Gastric lavage and administration of activated charcoal with a laxative can prevent more drug absorption if promptly given.
Diagnostics and labs monitoring
Psychosis and schizophrenia greatly impact the brain’s normal processes, which interfere with the ability to think clearly. When symptoms of these disorders are uncontrolled, patients may struggle to function in daily life. However, patients often thrive when properly diagnosed and treated under the close supervision of a psychiatric mental health practitioner.
For this Assignment, you will develop a study guide for an assigned psychotropic agent for treating patients with Schizophrenia Spectrum and Other Psychotic Disorders. You will share your study guide with your colleagues. In sum, these study guides will be a powerful tool in preparing for your course and PMHNP certification exam.
Resources
Be sure to review the Learning Resources before completing this activity.
Click the weekly resources link to access the resources.
WEEKLY RESOURCES
To prepare for this Assignment:
- Review this week’s Learning Resources, including the Medication Resources indicated for this week.
- Reflect on the psychopharmacologic treatments you might recommend for treatment of patients with Schizophrenia Spectrum and Other Psychotic Disorders.
- Research your assigned psychotropic medication agent using the Walden Library. Then, develop an organizational scheme for the important information about the medication.
- Review Learning Resource: Utah State University. (n.d.). Creating study guides. https://www.usu.edu/academic-support/test/creating_study_guides
- Links to an external site.
The Assignment
Create a study guide for your assigned psychotropic medication agents. Your study guide should be in the form of an outline with references, and you should incorporate visual elements such as concept maps, charts, diagrams, images, color coding, mnemonics, and/or flashcards. Be creative! It should not be in the format of an APA paper. Your guide should be informed by the FDA-approved and Evidenced-Based, Clinical Practice Guidelines Research but also supported by at least three other scholarly resources.
Areas of importance you should address, but are not limited to, are:
- Title page
- Description of the Psychopharmacological medication agent including brand and generic names and appropriate FDA indication uses
- Any supporting, valid and reliable research for non-FDA uses
- Drug classification
- The medication mechanism of action
- The medication pharmacokinetics
- The medication pharmacodynamics
- Mechanism of Action
- Appropriate dosing, administration route, and any considerations for dosing alterations
- Considerations of use and dosing in specific specialty populations to consider children, adolescents, elderly, pregnancy, suicidal behaviors, etc.
- Definition of Half-life, why half-life is important, and the half-life for your assigned medication
- Side effects/adverse reaction potentials
- Contraindications for use including significant drug to drug interactions
- Overdose Considerations
- Diagnostics and labs monitoring
- Comorbidities considerations
- Legal and ethical considerations
- Pertinent patient education considerations
- Reference Page
Note: Support your rationale with a minimum of five academic resources. While you may use the course text to support your rationale, it will not count toward the resource requirement. You should be utilizing the primary and secondary literature.
Reminder : The College of Nursing requires that all papers submitted include a title page, introduction, summary, and references. The Sample Paper provided at the Walden Writing Center provides an example of those required elements (available at https://academicguides.waldenu.edu/writingcenter/templates/general#s-lg-box-20293632). All papers submitted must use this formatting
By Day 7
You will need to submit your Assignment to two places: the Week 7 Study Guide discussion forum as an attachment and the Week 7 Assignment submission link. Although no responses are required in the discussion forum, a collegial discussion is welcome. You are encouraged to utilize your peers’ submitted guides on their assigned psychotropic medication agent for study.
Submission Information
Before submitting your final assignment, you can check your draft for authenticity. To check your draft, access the Turnitin Drafts from the Start Here area.
- To submit your completed assignment, save your Assignment as WK7Assgn_LastName_Firstinitial
- Then, click on Start Assignment near the top of the page.
- Next, click on Upload File and select Submit Assignment for review.
A Psychotropic Medication for Schizophrenia Spectrum and Other Psychosis Disorders
Risperidone is an atypical antipsychotic drug that psychiatrists prescribe for treating schizophrenia, bipolar disorder, and autism. It is also an adjunctive medication for treating other illnesses, including major depressive disorder, attention-deficit/hyperactivity disorder (ADHD), and substance use disorder (Alamo, 2022).
In this study guide, the indications, mechanism of action, pharmacokinetics, pharmacodynamics, dosing, administration route, side effects, contraindications, overdose, laboratory and diagnostic monitoring, comorbidities, legal and ethical considerations, and pertinent patient education considerations will all be discussed in detail. This guide provides a comprehensive overview of risperidone to ensure that it is prescribed and used safely and effectively.
Description of Psychopharmacological Medication Agent
Risperidone (brand name Risperdal) is an atypical antipsychotic drug approved by the FDA for treating schizophrenia spectrum disorders (including schizophrenia, schizoaffective disorder) and rapid-cycling bipolar disorder with or without psychotic features. It is also used off-label for conditions such as autism, major depressive disorder, ADHD, and substance use disorder.
Images of Risperidone sourced from https://www.drugs.com/image/risperidone-images.html
Drug Classification and Mechanism of Action
Risperidone is a benzisoxazole derivative, classified as an atypical antipsychotic. While typical antipsychotics, such as haloperidol, target only the dopamine receptors in the brain, risperidone also blocks serotonin receptors in the brain’s regulation of mood and behavior. This provides many benefits, such as decreased positive symptoms, improved social functioning, increased alertness and energy, improved attention, and enhanced cognitive functioning (Aucoin et al., 2020).
Additionally, risperidone is considered an “atypical” antipsychotic as it is thought to cause fewer side effects, such as those associated with typical antipsychotics, such as sedation, extrapyramidal symptoms, and weight gain. As such, risperidone is often prescribed in preference to other antipsychotics due to its greater potency and side effect profile. In addition, risperidone also has the advantage of treating multiple disorders without needing to be altered or augmented, as is often the case with other medications.
The Medication Pharmacokinetics
Risperidone is rapidly absorbed after oral administration, meaning that it is quickly absorbed and distributed throughout the body. It has a relatively short half-life of 10-20 hours compared to other antipsychotics, which makes it ideal for treating severe psychiatric disorders as it works quickly and has a relatively short duration of action in the body. The liver primarily metabolizes the medication, and the kidneys avidly excrete it.
This ensures that the drug is cleared from the body efficiently and reduces the risk of adverse reactions due to drug accumulation. The short half-life of risperidone also eliminates frequent dosing, as the drug can be taken as needed, allowing the patient more control over their medication regimen (Jablensky, 2022). Finally, the fast absorption and short half-life reduce the risk of drug interactions since the body can quickly clear the drug from the system, minimizing the risk of any interactions.
In conclusion, risperidone is rapidly absorbed, has a short half-life, is primarily metabolized by the liver, and is excreted mainly through the kidneys. These factors make it a suitable drug for treating severe psychiatric disorders and reduce the risk of interactions or adverse reactions.
Chemical structure of Risperidone-Image sourced from https://www.researchgate.net/figure/Chemical-structure-of-risperidone_fig7_332346771
The Medication Pharmacodynamics
Risperidone works by blocking (antagonizing) dopamine receptors (D2) in the brain, which are primarily involved in psychosis. Blocking or binding these receptors causes an increase in dopamine levels in the brain, which has an antipsychotic effect. At the same time, risperidone binds to other serotonin and dopamine (5HT2A and D2) receptors, which regulate behavior, emotional regulation, and mood.
This provides many benefits, such as better symptom control, improved social functioning, and fewer side effects (Jablensky, 2022). In addition, risperidone is beneficial in treating autism, major depressive disorder, ADHD, and substance use disorder.
Additionally, risperidone binds to two of the significant serotonin receptor subtypes (5HT2A and 5HT2C). This action reduces negative symptoms, such as flat affect and social withdrawal (Jablensky, 2022). It may also reduce hyperactivity in autistic patients and improve executive functioning and overall cognitive performance. Finally, risperidone also binds to receptors involved in appetite, metabolism, and cardiovascular regulation, which may help reduce weight gain associated with other antipsychotic medications.
| Risperidone blocks multiple receptors |
Additionally, Risperidone may have anti-anxiety effects due to its ability to block serotonin NMDA receptors, which modulate fear responses. Therefore, blocking these receptors is thought to have a calming effect that can be beneficial for people with psychosis (Jablensky, 2022). Taken together, Risperidone’s mechanism of action can lead to a reduction in psychotic symptoms, improved social functioning, and better overall control of emotions and behavior.
Appropriate Dosing, Administration Route, and Dose Adjustment
Risperidone is an atypical antipsychotic available in tablets, liquids, and injectables. The recommended adult dose is 4-8 mg daily, depending on the patient’s response. The dose of risperidone should be gradually increased until the desired therapeutic effect is reached. Adjusting the dose based on the patient’s response is essential, as this medication can lead to serious side effects if taken in excess. Additionally, patients should not self-adjust the dosage as it may lead to severe adverse reactions.
When prescribing risperidone, it is essential to consider the patient’s age, as the initial and maintenance doses should be adjusted accordingly. When dosing risperidone, clinicians should also consider other medications the patient may be taking, as some may interact with risperidone and lead to serious side effects (Luo et al., 2020). Finally, patients should be regularly monitored to ensure they respond to the medication appropriately.
Considerations of Use and Dosing in Specialty Populations
When prescribing risperidone, it is essential to consider the patient’s age and adjust the initial dose accordingly. In children and adolescents, the initial dose should be lowered to 1-2 mg, and further dose adjustments should be made based on the patient’s response. In elderly patients, the dose should be as low as possible due to the increased risk of adverse effects. The risks and benefits should be thoroughly weighed in pregnant women before prescribing risperidone.
It is important to note that risperidone should never be given to children two years and younger, and children under six need to be monitored closely for any adverse reactions (Luo et al., 2020). Additionally, the dose of risperidone should never exceed 8mg per day. Prescribers must consider all of these considerations to ensure the safe and effective use of risperidone.
Risperidone’s Half-Life and Important
Half-life is defined as the amount of time it takes for the concentration of a drug in the blood to be reduced to 50%. It is essential to understand the half-life of a drug because it is directly related to the drug’s duration of action. The half-life of risperidone is 10-20 hours, which is relatively short compared to other antipsychotics.
Side Effects/Adverse Reactions Potential
According to Müller (2018), risperidone can cause several adverse reactions, including nausea, vomiting, headache, dizziness, dry mouth, sedation, increased appetite, weight gain, and tremor. It may also cause extrapyramidal side effects, including restlessness, dystonia, akathisia, and Parkinsonian-like symptoms. Long-term use is also associated with the development of tardive dyskinesia, a neurological disorder caused by dopamine-blocking medications.
Contraindications and Drug-Drug Interactions
According to Alamo (2022), risperidone is contraindicated in patients with known hypersensitivity to risperidone or other formulation components. Additionally, it should not be used in patients with liver or renal disease, narrow-angle glaucoma, seizures, or Parkinson’s disease. It should also not be combined with certain medications due to drug-drug interactions, such as monoamine oxidase inhibitors (MAOIs).
Overdose Considerations
The clinical presentation of an overdose of risperidone would depend on the dose. Symptoms may include nausea, vomiting, dry mouth, excessive sweating, tachycardia, seizures, and coma. Treatment is often supportive and may include gastric lavage, activated charcoal, and close monitoring.
Diagnostics and Labs Monitoring
It is essential to monitor patients while they are taking risperidone. This includes performing blood tests to check for changes in blood count, electrolyte concentrations, or liver enzymes. Additionally, patients should be monitored closely for the development of extrapyramidal side effects.
Comorbidity Considerations
Comorbidities, including cardiovascular, cerebrovascular, and diabetes, should be considered when prescribing risperidone. Additionally, patients with bipolar disorder or substance use disorder should be monitored closely for any changes in mood or behavior.
Legal and Ethical Considerations
The prescribing of risperidone should be done within the scope of practice and according to the required guidelines. Informed consent should also be obtained from the patient before initiating the medication. Additionally, patients should be closely monitored for any adverse reactions or interactions. Informed consent leads to shared decision-making which allows the patient to be closely involved in creating the treatment plan.
This includes ensuring the patient understands the diagnosis, treatment options, and potential risks. It also includes providing the patient with relevant information about the medication and determining their personal preferences regarding treatment (Beck, 2021). Additionally, the patient should be educated about all treatment options and encouraged to participate in decision-making.
Patient autonomy is a critical ethical step when treating mental illness. In treating schizophrenia and other psychosis disorders, patient autonomy should be respected as much as possible. This begins with educating the patient on the diagnosis, the nature and effects of the medications, and the treatment plan. During this process, the patient should be allowed to ask questions about the medications, the reasoning behind their use, potential side effects, and realistic treatment expectations.
Legal considerations also play a role in treating schizophrenia and other psychosis disorders. In most states, antipsychotics require a prescription and authorization to use or administer the medication. This ensures that the medication is prescribed or administered according to the most up-to-date guidelines and is appropriate for the patient’s diagnosis and situation.
Furthermore, antipsychotics are widely regulated and must be prescribed and used according to the laws and regulations governing their use. In addition, prescribers and administrators should know the potential side effects of each medication, take steps to prevent abuse and misuse and document the correct dosage, administration route, and dosing history according to state regulations (Beck, 2021).
Pertinent Patient Education Considerations
It is essential for patients taking risperidone to adhere to their prescribed treatment plan and take the medication as directed. They should be cautious when increasing the dose and cautioned against taking more than the recommended dose. To ensure the optimal effects of the medication, risperidone should be taken with meals to help reduce nausea, and alcohol should be avoided while taking it (Lam, 2023).
Certain foods and activities should also be avoided, such as those high in tyramine, which may significantly increase blood pressure or heart rate (such as heavy exercise). Finally, patients should be made aware of the risks of the medication, such as an increased risk of diabetes and weight gain, and be advised to immediately link any changes in mental status, mood, or behavior to their healthcare provider.
Conclusion
Risperidone is a widely prescribed antipsychotic medication indicated for schizophrenia, bipolar disorder, and autism. It also has some non-FDA-approved uses, including major depressive disorder, ADHD, and substance use disorder. This study guide provides an overview of risperidone, including indications, mechanism of action, pharmacokinetics, pharmacodynamics, dosing, administration route, side effects, contraindications, overdose, laboratory and diagnostics monitoring, comorbidities, legal and ethical considerations, and patient education considerations. It is essential to consider all of these factors when prescribing risperidone to ensure the safe and effective use of the medication.
References
Alamo, C. (2022). Risperidone ISM as a new option in the clinical management of schizophrenia: a narrative review. Advances in Therapy, 39(11), 4875-4891. https://link.springer.com/article/10.1007/s12325-022-02299-8
Aucoin, M., LaChance, L., Clouthier, S. N., & Cooley, K. (2020). Dietary modification in the treatment of schizophrenia spectrum disorders: A systematic review. World Journal of Psychiatry, 10(8), 187. https://doi.org/10.5498%2Fwjp.v10.i8.187
Beck, N. S., Kim, D. S., & Dunn, L. B. (2021). Ethical Issues in Psychopharmacology. Focus, 19(1), 53-58. https://doi.org/10.1176/appi.focus.20200043
Jablensky, A. (2022). The diagnostic concept of schizophrenia: its history, evolution, and future prospects. Dialogues in Clinical Neuroscience. https://doi.org/10.31887/DCNS.2010.12.3/ajablensky
Lam, Y. F. (2023). Risperidone dosing schedule. The Brown University Psychopharmacology Update, 34(4), 2-3. https://doi.org/10.1002/pu.30995
Luo, C., Lencer, R., Hu, N., Xiao, Y., Zhang, W., Li, S., … & Gong, Q. (2020). Characteristics of white matter structural networks in chronic schizophrenia treated with clozapine or risperidone and those never treated. International Journal of Neuropsychopharmacology, 23(12), 799-810. https://doi.org/10.1093/ijnp/pyaa061
Müller, N. (2018). Inflammation in schizophrenia: pathogenetic aspects and therapeutic considerations. Schizophrenia Bulletin, 44(5), 973-982. https://doi.org/10.1093/schbul/sby024
Hello Oluwakemi! I enjoyed reading your discussion response this week, and I thought it was very insightful. I agree with the questions you asked in regards to understanding her depressive symptoms, however there are questions that need to be asked in regards to the patients insomnia as well. One of the questions, according to Stanford Medicine (2023), one of the questions for insomnia that can be asked if how long the patient sleeps each night (Stanford Medicine, 2023). This question allows the provider to understand the severity of the insomnia as well as the patients perception of the severity of the problem. In depressed patients, many times insomnia is a symptom that needs to be treated as well as the depression itself. Understanding the concepts around the insomnia such as behavioral aspects such as watching television or drinking caffeine, to simply not being able to fall asleep or stay asleep is very important in directing the treatment for this condition. Asking the patients questions about the insomnia gives a better understanding of the problem at hand.
Additionally, Duloxetine is a medication that is FDA approved for the treatment of major depressive disorder and not approved or used in an off label indication for insomnia (Stahl, 2021). This medication, while is fantastic for depressive symptoms, has side effects that are counterintuitive to this patients situation. This medication has the side effects of insomnia, which will worsen the problem at hand (Stahl, 2021). One of the other medications that can be used in the treatment of insomnia as well as depression is Clomipramine. Clomipramine is a medication that is used off label for both depression as well as insomnia (Stahl, 2021). Clomipramine can be started in lower doses for elderly patients to tolerate the medication better (Stahl, 2021). This medication does not have the side effect of insomnia, however this medication requires the patient to be monitored for weight gain, BMI, fasting plasma glucose, as well as cholesterol levels due to the side effect of increased appetite as well as weight gain (Stahl, 2021). Due to the patient having diabetes, it is very important to monitor these effects. It is also important to monitor the patients cardiac functioning during the course of this medication due to the side effects of QTc prolongation as well as arrhythmias (Stahl, 2021). Clomipramine also can cause orthostatic hypotension so blood pressure monitoring is essential (Stahl, 2021). Thank you for your post!
References
Stahl, S. M. (2021). Stahl’s essential psychopharmacology prescriber’s guide (7th ed.). Cambridge University Press.
Stanford Medicine. (2023). Diagnosis. Stanfordhealthcare.org. Retrieved January 12, 2024, from https://stanfordhealthcare.org/medical-
conditions/sleep/insomnia/diagnosis.html
Rubric
NURS_6630_Week7_Assignment_Rubric
| NURS_6630_Week7_Assignment_Rubric | |||
| Criteria | Ratings | Pts | |
| This criterion is linked to a Learning Outcome Create a study guide, in outline form with references, for your assigned medication. Incorporate visual elements such as concept maps, charts, diagrams, images, color coding, mnemonics, and/or flashcards. | 30 to >26.0 pts Excellent Point range: 90–100 The response is in a well-organized and detailed outline form. Informative and well-designed visual elements are incorporated. … Followed directions correctly by uploading assignment to Gradebook and submitted to the discussion forum area. 26 to >23.0 pts Good Point range: 80–89 The response is in an organized and detailed outline form. Appropriate visual elements are incorporated. … Partially followed directions by uploading assignment to Gradebook but did not submit to the discussion forum area. 23 to >20.0 pts Fair Point range: 70–79 The response is in outline form, with some inaccuracies or details missing. Visual elements are somewhat vague or inaccurate. … Partially followed directions by submitting to the discussion forum area but did not upload assignment to Gradebook. 20 to >0 pts Poor Point range: 0–69 The response is unorganized, not in outline form, or is missing. Visual elements are inaccurate or missing. … Did not follow directions as did not submit to discussion forum area and did not upload assignment to gradebook per late policy. | 30 pts | |
| This criterion is linked to a Learning Outcome Study guide completion elements addressed in Week 7 assignment area | 50 to >44.0 pts Excellent Point range: 90–100 The response thoroughly addresses all required content areas. 44 to >39.0 pts Good Point range: 80–89 The response adequately addresses all required content areas. Minor details may be missing. 39 to >34.0 pts Fair Point range: 70–79 The response addresses all required content areas, with some inaccuracies or vagueness. 34 to >0 pts Poor Point range: 0–69 The response vaguely or inaccurately addresses the required content areas. Or, three or more content areas are missing. | 50 pts | |
| This criterion is linked to a Learning Outcome Support your guide with references and research providing at least five evidence-based, peer-reviewed journal articles or evidenced-based guidelines. Be sure they are current (no more than 5 years old). | 10 to >8.0 pts Excellent Point range: 90–100 The response is supported by the 5 current, evidence-based resources from the literature. 8 to >7.0 pts Good Point range: 80–89 The response provides at least 4 current, evidence-based resources from the literature that appropriately support the study guide information. 7 to >6.0 pts Fair Point range: 70–79 3 evidence-based resources are provided to support the study guide, but they may only provide vague or weak justification. 6 to >0 pts Poor Point range: 0–69 2 or fewer resources are provided to support assessment and diagnosis decisions. The resources may not be current or evidence-based. | 10 pts | |
| This criterion is linked to a Learning Outcome Written Expression and Formatting – English writing standards: Correct grammar, mechanics, and proper punctuation | 5 to >4.0 pts Excellent Point range: 90–100 Uses correct grammar, spelling, and punctuation with no errors. 4 to >3.5 pts Good Point range: 80–89 Contains a few (1 or 2) grammar, spelling, and punctuation errors. 3.5 to >3.0 pts Fair Point range: 70–79 Contains several (3 or 4) grammar, spelling, and punctuation errors. 3 to >0 pts Poor Point range: 0–69 Contains many (≥ 5) grammar, spelling, and punctuation errors that interfere with the reader’s understanding. | 5 pts | |
| This criterion is linked to a Learning Outcome Written Expression and Formatting – The paper follows correct APA format for title page, headings, font, spacing, margins, indentations, page numbers, parenthetical/in-text citations, and reference list. | 5 to >4.0 pts Excellent Point range: 90–100 Uses correct APA format with no errors. 4 to >3.5 pts Good Point range: 80–89 Contains a few (1 or 2) APA format errors. 3.5 to >3.0 pts Fair Point range: 70–79 Contains several (3 or 4) APA format errors. 3 to >0 pts Poor Point range: 0–69 Contains many (≥ 5) APA format errors. | 5 pts | |
| Total Points: 100 | |||
Sample Answer for Study Guide For Medication Treatment Schizophrenia Spectrum And Other Psychosis Disorders Nurs 6630
Schizophrenia is characterized mainly by a clear sensory but a marked thinking disturbance. The psychotic disorder is highly linked with abnormalities of amine neurotransmitter function, especially dopamine. Antipsychotic medications, also called neuroleptic or major tranquilizers, are the first-line drug therapy for schizophrenia. They target the positive symptoms of schizophrenia, like hallucinations, delusions, and disorganized behavior. Antipsychotics act by controlling neurotransmitter dopamine and serotonin levels in the brain. They are classified into two major groups: Typical and Atypical. The antipsychotic effects of Typical antipsychotics owe their competitive blockage to dopamine receptors. Atypicals have fewer extrapyramidal adverse effects than the typical and block both serotonin and dopamine receptors. The purpose of this assignment is to develop a study guide for Risperidone.
Drug Description
- Risperidone is also called Risperdal.
- It is an Atypical neuroleptic medication.
- The FDA approves it for use in the USA in the treatment of:
- Schizophrenia in adults and children aged above 13 years.
- Bipolar I acute manic or mixed episodes as monotherapy in adults and children above 10 years (Álamo, 2022).
- Bipolar I acute manic or mixed episodes adjunctive with lithium or valproate in adults.
- Autism-associated irritability in children above five years.
Off-label uses include:
- Borderline personality disorder
- Delusional disorder
- Delirium
- Depression
- Brain injury
- Pedophilia
- PTSD
- Bipolar disorder
- Conduct disorder
- Lesch-Nyhan
- Tourette Syndrome
- Stuttering, movement disorders
- Developmental disorders
The medication mechanism of action
- Risperidone has a high affinity for serotonin type 2 (5-HT2) receptors.
- It binds to dopamine D2 receptors with 20 times lower affinity than that for 5-HT2 receptors (Zhao et al., 2022).
- It antagonizes alpha1-adrenergic, alpha2-adrenergic, and histaminergic receptors.
- Risperidone has a moderate affinity for serotonin type 1 receptors.
- Has a weak affinity for dopamine D1 receptors.
- No affinity for muscarinic, beta1-adrenergic, and beta2-adrenergic receptors (Zhao et al., 2022).
Pharmacokinetics
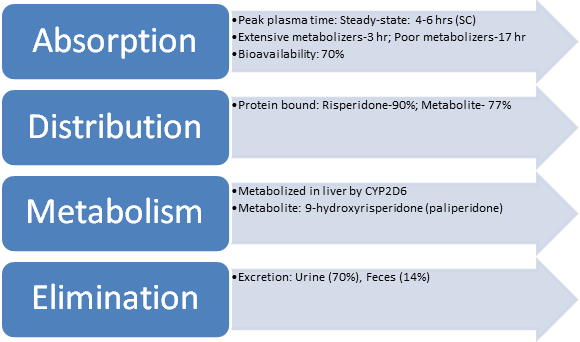
Pharmacodynamics
- Risperidone decreases dopamine neurotransmission for the five classes of dopamine receptors (D1 to D5).
- It reduces the hallucinations and delusions associated with schizophrenia by blocking dopamine receptors in the brain’s mesolimbic system (Zhao et al., 2022).
Appropriate dosing and administration route,
- Oral: Initial dose at 2 mg/day; Recommended target dosage of 2-8 mg/day OD or BD.
- The oral dose may be increased by 1-2 mg/day at intervals ≥24 hr.
- Intramuscular: 12.5-50 mg into the deltoid or gluteal muscle every 2 weeks; The dose should not be adjusted more often than every 4 weeks.
- Subcutaneous: 90 mg or 120 mg SC once monthly.
Considerations For Dosing Alterations
- Renal Impairment: If Creatine clearance is below 30, PO Risperidone should be initiated at 0.5 mg BD.
- It can be increased by up to 0.5 mg BD to a max of 1.5 mg BD.
Considerations of use and dosing in specific specialty populations
Geriatrics: Lower initial doses are recommended and should be adjusted more gradually.
PO initial dose at 0.5 mg q12hr; IM: 12.5-25 mg (Álamo, 2022).
Pediatrics >13 years: Initiated at lower doses of 0.5 mg/day PO in the morning or evening.
Half-life: This is the time taken for the plasma or blood level of a drug to fall by half.
- Half-life is determined by drug distribution, metabolism, and excretion.
- Half-life is important because drugs that have a short half-life stay in the body for a shorter period and thus have a shorter duration of action (Andrade, 2022).
- Drugs with a short half-life thus need to be administered more frequently.
- Drugs with a long half-life stay longer in the body and thus have a longer duration of action (Andrade, 2022).
- These drugs can conveniently be dosed once a day or less frequently.
- Extensive Risperidone metabolizers have a half-life of 3 hrs (parent and metabolite combined).
- Poor metabolizers have a half-life of 20 hrs (parent and metabolite combined).
Side effects/adverse reaction potentials
- The most significant side effects are Weight gain, metabolic changes, and sedation.
- It is associated with extrapyramidal symptoms (EPS): Acute dystonia, tardive dyskinesia, akathisia, and parkinsonian features (Hodkinson et al., 2021).
- Neuroleptic malignant syndrome (NMS) is a serious side effect of Risperidone.
- Other side effects: Somnolence, Insomnia, Agitation, Anxiety, Headache, Rhinitis, Fatigue, Increased appetite, Vomiting, Drooling, and Urinary incontinence (Hodkinson et al., 2021).

Contraindications for use and drug-to-drug interactions
- Risperidone is contraindicated in patients with known allergy/hypersensitivity to Risperidone or paliperidone (Hodkinson et al., 2021).
- Contraindicated in dementia-related psychosis due to increased risk of death.
Overdose Considerations
- Risperidone overdose is life-threatening.
- Patients with risperidone overdose should be monitored for hypotension, sedation, and respiratory depression.
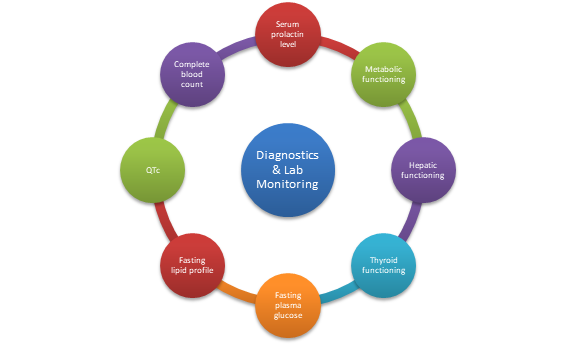
Diagnostics and labs monitoring
- Monitoring plasma concentrations for Risperidone is strongly recommended.
- Monitor for leukopenia/neutropenia and agranulocytosis.
- Monitor complete blood count frequently in the first few months of therapy in patients with a history of low WBC count (Álamo, 2022).
- Specific parameters to be monitored: Serum prolactin level, hepatic functioning, metabolic functioning, thyroid functioning, blood pressure, fasting plasma glucose, fasting lipid profile, and QTc (Álamo, 2022).
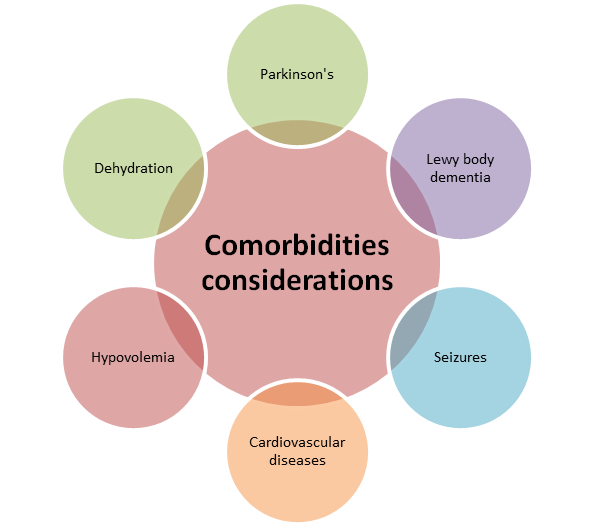
Comorbidities considerations
- It should be administered with caution in patients with a history of Parkinson’s disease, Lewy body dementia, seizures, cardiovascular disease, hypovolemia, and dehydration (Álamo, 2022).
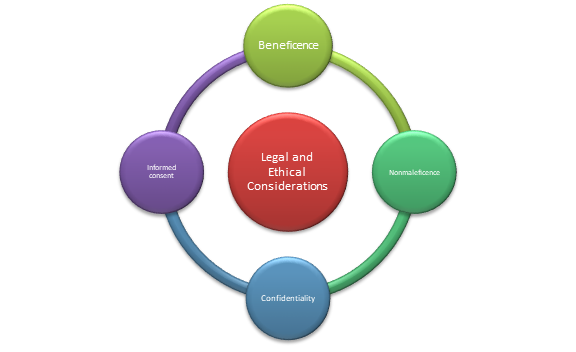
Legal and ethical considerations
- The prescribing clinician should consider legal and ethical factors of beneficence, nonmaleficence, informed consent, and confidentiality when prescribing Risperidone (Hodkinson et al., 2021).
- Beneficence is fostered by prescribing the drug when evidence supports its efficacy and benefits in treating schizophrenia in patients.
- Nonmaleficence is upheld by examining the associated side effects of Risperidone and ensuring the benefits outweigh the possible harm.
- The clinician should obtain consent from the patient by explaining the mechanism of action, benefits, and potential side effects of Risperidone before starting treatment (Hodkinson et al., 2021).
- The clinician should maintain the confidentiality of the patient’s diagnosis and treatment and seek consent before sharing the information with other providers.
Pertinent patient education considerations
- Patient education with regard to Risperidone includes informing the patient of the drug’s benefits in alleviating schizophrenia symptoms and possible side effects.
- Patients should be educated on extrapyramidal symptoms and signs of NMS (Hodkinson et al., 2021).
- They should be instructed on the action to take when serious side effects occur.
Conclusion
Risperidone is an Atypical neuroleptic used to treat schizophrenia in adults and children above 13 years. It is also FDA-indicated to treat Bipolar 1 acute manic or mixed episodes and Autism-associated irritability in children above 5 years. It exhibits its therapeutic effects by blocking serotonin and dopamine receptors. The most significant side effects of Risperidone are weight gain, metabolic changes, and sedation. Neuroleptic malignant syndrome and Extrapyramidal symptoms are serious side effects of Risperidone.
References
Álamo, C. (2022). Risperidone ISM as a New Option in the Clinical Management of Schizophrenia: A Narrative Review. Advances in therapy, 39(11), 4875–4891. https://doi.org/10.1007/s12325-022-02299-8
Andrade, C. (2022). The practical importance of half-life in psychopharmacology. The Journal of Clinical Psychiatry, 83(4), 41940. https://doi.org/10.4088/JCP.22f14584
Hodkinson, A., Heneghan, C., Mahtani, K. R., Kontopantelis, E., & Panagioti, M. (2021). Benefits and harms of Risperidone and Paliperidone for treatment of patients with schizophrenia or bipolar disorder: a meta-analysis involving individual participant data and clinical study reports. BMC medicine, 19(1), 195. https://doi.org/10.1186/s12916-021-02062-w
Zhao, M., Ma, J., Li, M., Zhu, W., Zhou, W., Shen, L., Wu, H., Zhang, N., Wu, S., Fu, C., Li, X., Yang, K., Tang, T., Shen, R., He, L., Huai, C., & Qin, S. (2022). Different responses to risperidone treatment in schizophrenia: a multicenter genome-wide association and whole exome sequencing joint study. Translational psychiatry, 12(1), 173. https://doi.org/10.1038/s41398-022-01942-w

Don’t wait until the last minute
Fill in your requirements and let our experts deliver your work asap.